ABMI
Main menu:
- Home
- About ABMI
- Clinical Focus
- Monitoring Software
- TCD Systems
- Clinical Studies
- News/Investors
- Contact Us
Miniature, USB-Powered TCD System: The Embol-ID
TCD Systems
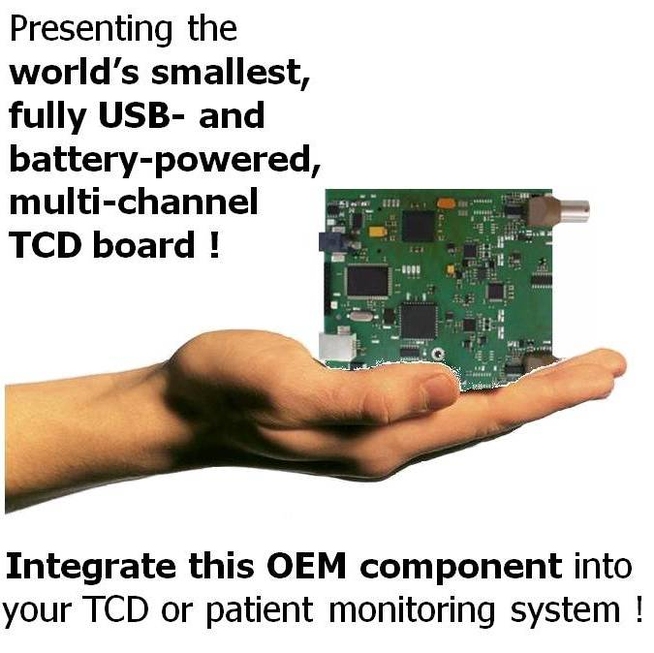
Embol-ID is ABMI's complete (hardware + software) system for brain flows and embolization monitoring. It identifies in real time and sizes brain emboli, which cause cerebral ischemia and stroke, e.g. during cardio-vascular surgery.
Embol-ID allows surgeons and neurologists:
- to assess the neurological risk and to adapt the surgical or transcutaneous procedure,
- to perform focused drug deliveries by providing clear therapeutic indications.
Embol-ID is provided on an OEM basis.
As for the other products, the Embol-ID can be delivered either as a complete system with PC or as a USB electronic board with the adapted software.
The Embol-ID can be provided in two versions:
- The first version is tailored for use in the operation room, and aimed at improving the neuroprotection of cardio-vascular surgical patients. It is not designed to work independently of the attached computer.
- The second version is equipped with an additional power pack and mass memory, in order to perform ambulatory patient monitoring. Such "ambulatory" capabilities are needed for OR monitoring also, typically for keeping the patient monitored when he/she is transferred form the OR to the ICU.
The Embol-ID is thus a full, real-time TCD monitoring system intended for embolization monitoring in the OR.
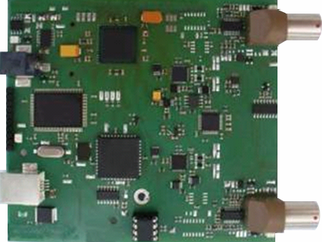
The Embol-ID Hardware
The Embol-ID hardware is a digital, pulsed-wave TCD hardware. The board is low consumption, fully USB-powered, with battery operation possible. It drives two 2 MHz transcranial probes, and is fitted to the Embol-ID software. It features dual probes, and on-board memory for data storage (e.g. for the case of ambulatory patient monitoring). This very small board (approx. 10 x 10 cm) is depicted here.
The Embol-ID Software
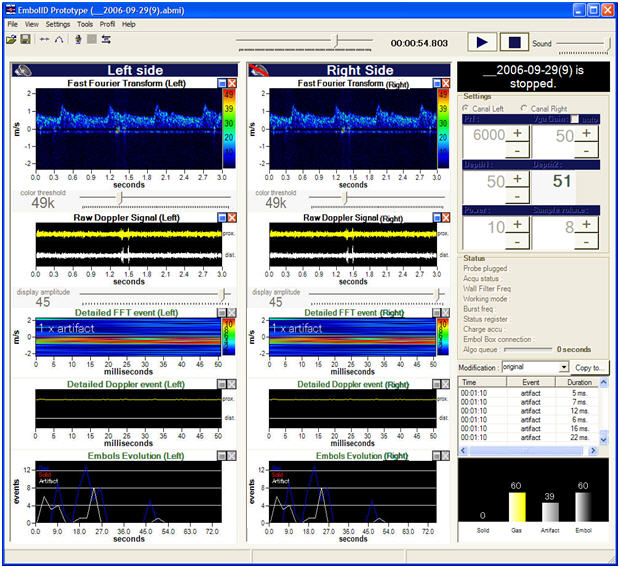
The Embol-ID software features, as shown on the interface printout:
- Dual side FFT of the blood flows,
- Dual side display of the raw Doppler signal (the so-called "audio signal"), at two different depths,
- Dual side detailed FFT and detailed raw Doppler signal display for the detected high intensity events,
- Dual side timely evolution of the numbers of detected artifacts, solid emboli, and gaseous emboli.
Furthermore, the Embol-ID software drives the TCD board, and allows for real-time analysis of embolization via the integration of ABMI's signal processing algorithmics.
VIEW A VIDEO OF THE Embol-ID MONITORING A HEALTHY SUBJECT